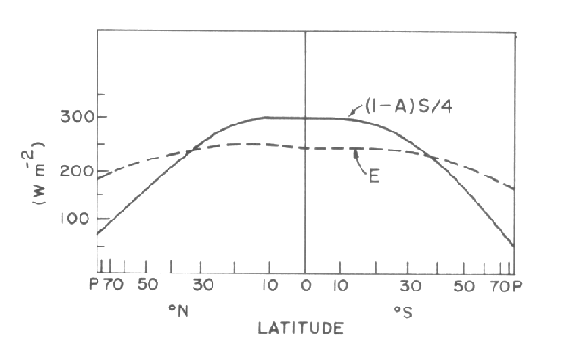

The poleward transport of energy by the atmosphere and oceans moderates the temperature contrasts between equator and the winter pole and makes the earth a much more livable planet than it would be if the mean temperature in each latitude belt were determined entirely by radiation. They balance the energy budget, removing the surplus at low latitudes and delivering it to higher latitudes where it balances the deficit. The transport mechanism can be understood as follows. On average, poleward moving parcels of air and water contain relatively large amounts of internal energy: i.e., they are relatively warm compared to other parcels that lie along the same latitude circle. During the time that they spend in high latitudes, they tend to lose energy (i.e., cool off) so that by the time they return to the tropics they contain less energy than they did on the poleward leg of their trajectory. Conversely, while they are in the tropics, they tend to gain energy (warm up), so that by the time they go poleward again, their energy supply is replenished.
If the temperature distribution at the earth's surface were entirely determined by radiative transfer, the tropics would be substantially warmer than they are today and temperatures in the polar cap regions would approach absolute zero (-273 C) every winter, when the sun disappears over the horizon. The poleward advection of energy by the atmosphere and oceans moderates the temperature contrasts between equator and the winter pole. On average, poleward moving parcels of air and water contain relatively large amounts of internal energy: i.e., they are relatively warm compared to other parcels that lie along the same latitude circle. During the time that they spend in high latitudes, they tend to lose energy (i.e., cool off) so that by the time they return to the tropics they contain less energy than they did on the poleward leg of their trajectory. Conversely, while they are in the tropics, they tend to gain energy (warm up), so that by the time they go poleward again, their energy supply is replenished.
The role played by parcels of air and water in transporting energy into high latitudes is analogous to the one played by my dog in transporting dirt into the house on a wet day. When she comes in from the garden, she is covered with mud. While she is in the house, she gets rid of most of the dirt while walking around the carpets, brushing against the walls, etc. By the time she goes out, she is relatively clean and dry, ready to pick up another load. If it were not for her, the house would stay cleaner and more of the mud would stay in the garden.
Just as my dog's the trips in and out of the house seem purposeful, at least to her, the motions responsible for the poleward transport of energy have distinct forms of organization. We have already considered one of these forms: the flow of the surface winds around the subtropical anticyclones over the oceans during summertime. The poleward moving air on the western sides of the anticyclones that passes over cities like Washington, DC, Tokyo, and Buenos Aires, is warm (often hot ) and humid: i.e., it contains large amounts if internal energy and latent heat.
Another kind of atmospheric motion system that transports energy poleward is what meteorologists call 'baroclinic waves': eastward moving disturbances in middle latitudes that are responsible for the pronounced day-to-day weather changes that we observe, particularly during wintertime. Baroclinic waves are characterized by alternating incursions of cold, dry polar air masses into the subtropics and incursions of warm humid subtropical air masses into higher latitudes. The advancing air masses are often marked by sharp fronts linked to developing 'cyclones' (wind systems in which the air circulates in the same sense as the earth's rotation: counterclockwise in the Northern Hemisphere). Most of the rain and snow that falls in the extratropics is associated with the passage of these cyclones and their attendant fronts. The warm air masses precede the cyclones and the cold air masses usually follow.
The ocean circulation accounts for a substantial fraction of the poleward heat transport required to account for the imbalance between incoming solar radiation and outgoing longwave radiation. Two fundamentally different kinds of circulations contribute: the wind driven gyres in the top few hundred meters of the oceans and the 'thermohaline circulation' which extends to the bottom of the world ocean.
The gyres are driven by the the subtropical anticyclones in the surface wind field and they circulate in the same direction: clockwise in the Northern Hemisphere and counterclockwise in the Southern Hemisphere. The Gulfstream in the western North Atlantic (off the coast of the eastern United States) and the Kuroshio Current in the western North Pacific (off Japan) represent the warm northward branches of these gyres and the more diffuse southward flowing waters in the Northeast Pacific (off the Washington coast) and in the North Atlantic (off the coast of Europe) represent the cooler southward flowing branches. There are analogous gyres, that circulate in the opposite direction, in the South Atlantic and the South Pacific.
TRY TO FIND A LINK" PETER RHINES??
The thermohaline circulation functions like a
gigantic conveyor belt carrying warm surface water northward to the ice
edge in the Atlantic,
where it cools and sinks to the bottom of the
ocean and flows southward along the bottom of the ocean, crossing the equator
into the southern hemisphere and subsequently flowing into the other oceans
through the Drake Passage south of the tip of South America and through
the broader passage south of Africa. This 'bottom water' eventually
finds its way back the upper layers of the ocean where it's warmed by the
sun. It returns to the Atlantic in surface currents, thereby completing
its circuit around the conveyor belt.
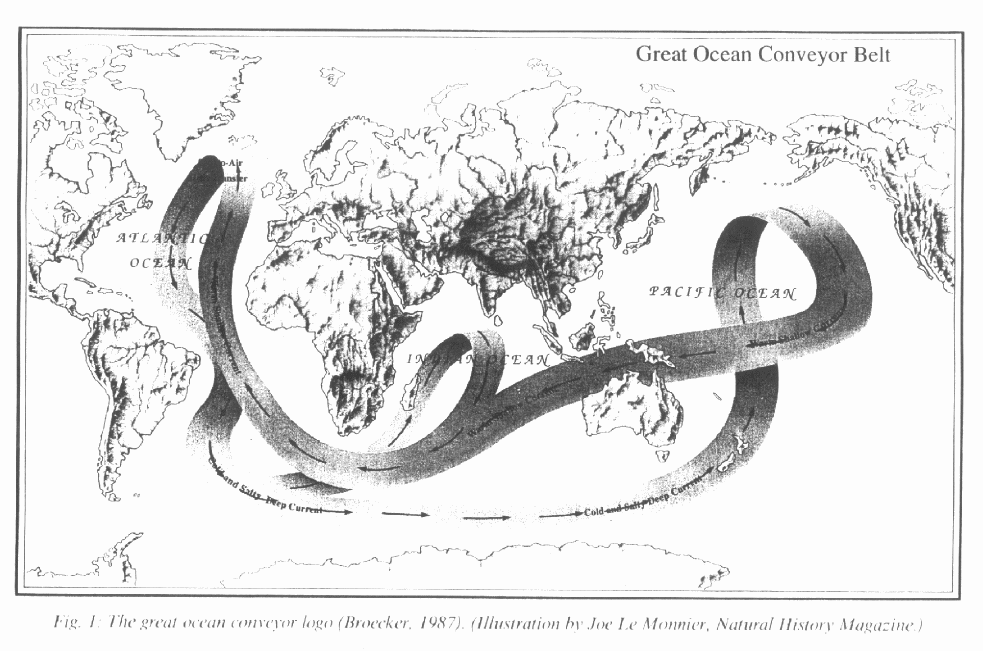
Since the thermohaline circulation tends to be
focused on the North Atlantic, that's where it performs virtuallu all of
its poleward heat
transport. Why is it the North Atlantic
as opposed to the North Pacific? Because the North Atlantic is much deeper
and extends poleward to the ice edge, whereas the North Pacific is walled
in by land masses.
Contrasts in both the temperature and salinity
(saltiness) of the ocean water play a role in driving the 'thermohaline
circulation' : Hence, the
name. Both properties affect the
density of the water. Other things being equal, cold water (above
4 C) is denser than warm water and more saline water is denser than fresher
water. Temperature is the dominant factor in determining the density
except where temperatures are close to freezing, in which case salinity
is dominant. It is notable that between 0 and 4 C, density actually
decreases with temperature: that's why ice floats on top of liquid water
rather than sinking to the bottom. Bottom water, which occupies roughly
the bottom half of the 'world ocean' is at a temperature close to 4 C,
the temperature at which water of a given salinity is densest.
Ordinarily, the water in a vertical column is 'stably stratified' with denser water on the bottom and lighter water on top. The formation of bottom water requires that surface waters acquire a density large enough to sink to the bottom of the ocean (i.e., that they become denser than the intermediate waters). We know that this actually happens in the North Atlantic because bottom waters contain chemical tracers such as CFC's that are known to have been in more recent contact with the atmosphere than the intermediate waters in the column. Two processes play a role in increasing the salinity of the surface waters along the ice edge in the North Atlantic: evaporation and brine rejection.
Evaporation invloves a loss of fresh water to the atmosphere, leaving saltier water in its wake. Conversely, precipitation freshens the surface waters. Evaporation is locally very strong along the ice edge where cold, dry air masses often emerge from over the Arctic Pack ice and flow out over the relatively warm surface waters picking up large quantities of heat and moisture from the underlying water. Brine rejection is an essential part of sea ice formation: the water incorporated into the ice is completely fresh: the salt in the sea water is rejected, creating pockets of very saline water along the ice edge. Through these mechanisms, water can become dense enough to sink all the way to the bottom of the ocean.
Northern Europe is conspicuously warm for its latitude. For example. England, whose climate is much like Seattle's is at the same latitude as the expanses of stunted boreal forest and tundra found in Labrador and northern Quebec. The thermohaline circulation is believed to play an important role in maintaining that anomalous warmth. Were it to weaken or come to a halt for any reason, the climate of Northern Europe could become considerably colder, particularly during winter. Ocean models of the thermohaline circulation suggest that its intensity may be quite sensitive to the salinity of the surface water. In particular, if it were possible by some mechanism to create a cap of anomalously fresh water in the region of the ice edge, the formation of bottom water would effectively be shut down, and the thermohaline circulation would come to a halt, and with it the northward flow of warm water. In the absence of the poleward heat transport by the thermohaline circulation, the surface waters along the ice edge would cool and freeze, and the pack ice would expand. A pool of anomalously fresh, cold water did, in fact, appear suddenly in this region during the 1970's and was tracked for almost a decade as it drifted first westward and then later eastward with the surface currents.
LINK TO CALVIN HOME PAGE
Another kind of atmospheric motion system that
transports energy poleward is what meteorologists call 'baroclinic waves':
eastward moving disturbances in middle latitudes that are responsible for
the pronounced day-to-day weather changes that we observe, particularly
during wintertime. Baroclinic waves are characterized by alternating incursions
of cold, dry polar air masses into the subtropics and incursions of warm
humid subtropical air masses into higher latitudes. The advancing air masses
are often marked by sharp fronts linked to developing 'cyclones' (wind
systems in which the air circulates in the same sense as the earth's rotation:
counterclockwise in the Northern Hemisphere). Most of the rain and
snow that falls in the extratropics is associated with the passage of these
cyclones and their attendant fronts. The warm air masses precede
the cyclones and the cold air masses usually follow.
Where did the atmosphere come from? The atmosphere is believed
to have originated from the expulsion of volatile substances (i.e., compounds
capable of existing in a gaseous state) that were part of the cloud of
debris orbiting the sun, from which the planets formed. The volcanic
eruptions of the past few decades have provided samples of the kinds and
relative and total amounts of these substances. First and foremost
is water vapor, which accounts for ~85% of the total; then comes CO2
and loss oxidized carbon gases CO and CH4, with ~10%.
The remainder is a mix of sulfur compounds, N2 and NH4
(ammonia)
and volcanic ash, laced with traces of other gases. Based on estimates
of the amounts of volatiles expelled by the major volcanic eruptions during
the 20th century, the average amount of water released into the atmosphere
per year, if sustained over the 4.5 billion year lifetime of the earth,
should have been enough to fill the
oceans many times over. [LINK TO PROBLEM / SOLUTION] Hence, it
is believed that the oceans leak: i.e., that the water molecules in the
steam and ash clouds emanating from today's volcanoes have been 'recycled'
through the atmosphere and oceans, the earth's crust, and injected back
into the atmosphere by volcanic eruptions many times in the past.
In view of the marked difference between the makeup of today's volcanic eruptions and the present composition of the atmosphere, the question arises: how does a mix of gases consisting of mostly water vapor and carbon compounds get converted into one in which the dominant constituents are N2 and O2? To answer this question we have to identify the 'sinks' or repositories for the water vapor and carbon molecules and to identify the 'sources' of the N2 and the O2.
Water vapor condenses into clouds and rains out of the atmosphere within
days of the time that it is injected. The amount retained in the
atmosphere bears no relation to the amount put in: it is determined
solely by the temperature and distribution of relative humidity within
the atmosphere. Water vapor could become a major constituent of the
earth's atmosphere only if the surface air temperature rose to the boiling
point of water (in which case the oceans would literally boil away), or
if the other constituents somehow disappeared.
There are two major pathways by which carbon compounds injected into the atmosphere by volcanic eruptions has been removed: both involve chemical reactions in which biology plays a role.
Green plants on land and phytplankton in the sea convert carbon dioxide and water into chlorophyll through the photosynthesis reaction
H2O + CO2 + photon -> {CH2O} + O2
where the {CH2O} is the building block for the organic molecules
in plants. Photosynthesis requires sunlight. The "photon" in the
reactions involves a discrete amout of energy, derived from solar radiation
at visible wavelengths, which is necessary to form the chemical bond.
The photosynthesis reaction also yields a molecule of O2 as
a 'waste product'.
The respiration and decay reaction
{CH2O} + O2 -> H2O + CO2
undoes the work of photosynthesis. Like the burning of fossil fuels, it involves the oxidation of {CH2O}, returning carbon to the atmosphere. Hence, plants that grow and subsequently decay do not result in any net removal of carbon from the atmosphere over their complete life cycle. The products of photosynthesis outlive the organism that performs it only if the decay is forestalled by burial (or sinking in an anoxic (oxygen free) region of the ocean) so that the {CH2O} does not come into contact with oxygen. Oxidation of the carbon containing molecules can be postponed indefinitely if they are incorporated into sediments as 'fossil carbon'.
The other pathway for the removal of carbon from the atmosphere involves the formation of calcium carbonate (limestone) in ocean sediments. Several different chemical reactions are involved in this process, whose net effect is to combine a calcium (Ca) ion in sea water with a dissolved carbon dioxide molecule, and an oxygen atom to form CaCO3. This reaction also involves living organisms (i.e., predominantly microscopic 'forams' whose shells settle to the bottom of the ocean when they die and are incorporated into sediments. In contrast to photosynthesis, which liberates oxygen, carbonate formation sequesters it in sediments.
The reservoir of organic carbon and carbonates in land and ocean sediments is large enough to account for the mass of carbon believed to have been injected into the atmosphere by volcanic eruptions over the lifetime of the earth. The only constituent of volcanic emissions that has remained in the atmosphere in appreciable quantities is N2, which is much less soluble in the oceans than CO2 and has not been removed as effectively.
The photosynthesis reaction discussed above is believed to have been the major source of free oxygen on the earth system. Over the lifetime of the earth it must have produced enough O2 to account, not only for the amount present in the atmosphere, but also for the much larger amounts required to form all the limestone in ocean sediments and to oxidize the iron and other metals in the earth's crust. Noting that the photosynthesis reaction liberates one molecule of O2 for each carbon atom incorporated into living plants, it follows that the amount of numer of oxygen molecules liberated must equal the number of carbon atoms buried in sediments. Based on estimates by geologists, the reservoir of fossil carbon in sediments is, in fact, large enough to account for the required production of oxygen.The buildup of oxygen in the atmosphere poses an apparent dilemma. Chlorophyll producing plants could not survive were it not for the presence of the stratospheric ozone layer, which shields them from the harmful effects of the ultraviolet radiation in the solar spectrum. Yet the oxygen that the plants have produced over the course of time is the 'raw material' for the chemical reactions that give rise to the ozone layer that protects them. So how did the process of oxygen and ozone production get started in the first place? Marine biota living far enough below the ocean surface so that the water shields them from solar ultraviolet radiation are believed to have been instrumental in producing oxygen until the amount in the atmosphere became sufficient to support the beginnings of an ozone layer. The buildup of the ozone layer would have enabled marine biota to gradually expand upward toward the ocean surface, where they had access to more solar visible radiation needed for chlorophyll production. The upward expansion of marine biota, in turn, would have increased the rate of production of oxygen and ozone: a kind of positive feedback, which eventually led to a sudden breakthrough, when life expanded onto land and the oxygen concentration in the atmosphere rose sharply to very close to its present level. The sudden rise in oxygen concentration must have been a catastrophe for many previously existing life forms that had evolved in an anoxic environment. Fossil evidence indicates that this dramatic turning point in the history of the earth took place about half a million years ago. Hence, the formation of the ozone layer, expansion of life onto land, and the buildup of oxygen in the atmosphere to present levels are relatively recent developments in the earth's history.
More about carbon in the earth system
In order to understand the implications of the burning of fossil fuels we need to learn more about the various reservoirs of carbon in the earth system, their capacities, and the ways in which carbon is exchanged between them. In conducting this inventory we will use gigatons (billions of metric tons; 10 to the 12th kilograms) of carbon as a unit. We take account of just the carbon atoms in molecules of CO2, CaCO3, etc., not the other atoms.
______
PROBLEM: estimate the number of gigatons of carbon in the earth's atmosphere
based upon the following information: TO BE ADDED NEXT YEAR--
_______
Here are some rough estimates of the amount of carbon in some of the
reservoirs. Note that the reserviors are not mutually exclusive;
some of them partially overlap.
| atmosphere
marine biota land vegetation soils and organic debris total land biosphere dissolved in ocean marine sediments(CaCO3) fossil carbon fossil fuels |
3 610 1,580 2,190 40,000 20,000,000 36,000,000 7,500 |
The atmosphere contains only a minute fraction of the carbon in the earth system the (storage in sediments is many orders of magnitude larger). The fossil fuel reservoir is about twice as large as the atmospheric reservior. Hence, if all the fossil fueld were instantaneosly buned and the carbon dioxide injected into the atmosphere without anything else happening, the atmospheric concentration of CO2 would increase by about a factor of 10, ao that instead of being about 375 parts per million by volume it would be nearly 4000 parts per million; 4 parts per thousand, or 0.4%. Not even the most pessimistic scientists in the community are predicting increases of that magnitude, for reasons that will become clear later on.
Why is the fossil fuel reservoir so small in comparison to the total amout of fossil carbon buried in sediments? Because it's too dispersed use: the amount of energy required to mine and process it would exceed that gained by burning it (not to mention the environmental degradation caused by the mining). The fact that most of the carbon supply is inaccessable ensures that only a minute fraction of the oxygen on the earth system can possibly be consumed by burning fossil fuels. Humankind is in no danger of suffocating itself by using up all the oxygen in the atmosphere.
The amount of carbon injected into the atmosphere each year by the burning of fossil fuels can be estimated to within about 10% on the basis of fuel production consumption statistics. It amounts to ~5 gigatons (Gt) per year, less than one thousandth of the estimated reservoir of exploitable fossil fuels (natural gas, oil, and coal). In other words, at the current rate of consumption, the know supply of fossil fuels would last over a thousand years. However, the consumption rate is expected to increase substantially as world population continues to grow and per capita energy consumption in third world nations such as China and India increases. For example, energy production (from all sources combined) would have to triple in order to bring 60% of the current world population up to a consumption rate of 1,500 kW per year per capita, about 10% of the current rate in the U.S..
Fossil fuel burning isn't the only thing that causes atmospheric CO2
concentrations to vary. It is estimated that 60 Gt of carbon are
transferred back and forth between the atmosphere and the terrestrial (land)
biosphere each year, through photosynthesis and respiration / decay. One
can see clear evidence of this cycling back and forth in time series of
atmospheric concentrations at Northern Hemisphere observing stations such
as Mauna Loa (LINK TO FIGURE). Each year the concentrations drop
steadily through spring and summer These changes, which are observed
each year at nearly the same time, reflect the 'breathing' of the Northern
Hemisphere terrestrial biosphere. The falloff during spring and summer
is due photosynthesis, which is increasing the biomass of most plants species
in temperate and high latitudes. When leaves and many grasses decay
in autumn, the carbon that they fixed is returned to the atmosphere. The
CO2
record for the South Pole station also shown in the figure does not
exhibit a spring / summer drawdown. The difference between the two
time series reflects the much larger land area of the Northern Hemisphere.
________________
PROBLEM: By how many ppm does CO2 at Mauna Loa drop during spring and
summer of a typical year? Assuming that the Mauna Loa time series
is typical of Northern Hemisphere stations, estimate the decrease in the
mass of atmospheric carbon during these months.
SOLUTION: TO BE ADDED
______________
Upon close inspection it is apparent that the amplitude of the spring
/ summer drawdown in atmospheric CO2 concentration has increased slightly
during the past few decades, which is suggestive of a more pronounced 'greening'
of the Northern Hemisphere biosphere during the growing season in recent
years.
Apart from the difference in the annual drawdown, the South Pole and Mauna Loa CO2 time series track quite closely, and they exhibit virtually identical upward trends of ~1 ppm (~0.3%, equivalent to an increase in mass of ~2,5 gigatons) per year. Apparently, the rate of mixing of CO2 between Northern and Southern hemispheres is fast enough to keep the annual means of the two time series very close together, but slow enough to allow for the pronounced differences in the seasonal behavior in the two hemispheres.
The rate of injection into the atmosphere and the rate of increase in the mass of carbon in the atmosphere are both based upon solid information, yet they differ by about a factor of 2. Hence, there is good raeson to believe about half the carbon injected into the atmosphere by the burning of fossil fuels is finding its way into other reservoirs, reducing the potential for greenhouse warming (at least in the short term). Scientists have used models of carbon transport in order to try to infer how hich of the missing carbon ends up in the terrestrial biosphere and how much is dissolving in the oceans. The apparent 'greening' of the Northern Hemisphere terrestrial biosphere noted above supports the notion that land plants are taking up some of the slack. On the other hand; it is clear that the destruction of old growth forests in areas such as Brazil, Malaysia, western canada and Alaska are reducing the storage in the terrestrial biosphere. Which of these two processes dominates is not clear.
Upon close inspection it is apparent that the amplitude of the spring / summer drawdown in atmospheric CO2 concentration has increased slightly during the past few decades, which is suggestive of a more pronounced 'greening' of the Northern Hemisphere biosphere during the growing season in recent years. [http://www.mganet.org/WWWdisplay.cgi?49020355]
Ozone is of critical importance to life on earth because it protects it from the potentially lethal effects of ultraviolet radiation with wavelengths shorter than 0.31 microns. In order to understand why this radiation is dangerous and how ozone protects life from it, it is necessary to talk about a property of radiation has not yet been touched on in these notes. Instead of thinking about radiation as electromagnetic waves with a spectrum of wavelengths, it is equally valid to think of it as a stream of packets or "photons" of energy that come in a spectrum of sizes, where the amount of energy contained in a given photon is inversely proportional to the wavelength of the equivalent electromagnetic wave: i.e., the shorter the wavelength, the more energetic the photon.
Over 98% of the energy in the spectrum of solar radiation is at visible and near infrared wavelengths, but around 1.75% is at ultraviolet wavelengths, where the energy level of the individual photons is high enough to damage the cells of biological organisms that are exposed to them. It's analogous to having a few very big drops ir hailstones among a spectrum of raindrops, which account for only a small fraction of the total mass of the rain, but because of their own large mass they might be capable of causing certain things to happen that are qualitatively different from the effects of the much more numerous smaller drops.
The more energetic 'hard ultraviolet' radiation from the sun: i.e., the radiation with wavelengths shorter than 0.2 microns, is capable of dissociating atomic oxygen (i.e., breaking it up into a pair of oxygen atoms). This reaction takes place high up in the stratosphere, shielding lower layers from radiation in this wavelength range. The 'soft ultraviolet' radiation with wavelengths longer than 0.2 microns isn't energetic enough to "photodissociate" O2 or any of the other major atmospheric constituents. Were it not for the ozone layer, it would penetrate through the atmosphere to the earth's surface.
The atomic oxygen created by photodissociation of O2 plays a critical role in the formation of the ozone layer. When an oxygen atom undergoes a three-way collision with an O2 molecule and any other molecule, the O and O2 bond to form an ozone molecule. In symbols:
O + O2 + M -> O3 + M (1)
where the M represents the third molecule, whose role in the rection is simply to absorb the excess energy released when the O and O2 bond together. Without it, the ozone molecule formed in the collision would go away with so much energy that it would immediately break apart.
Ozone molecules are not as tightly bonded as O2 molecules so it doesn't take as much energy to break them apart. Soft ultraviolet radiation with wavelengths shorter than 0.31 microns is energetic cause the reaction:
O3 + photon -> O + O2 (2)
In the process of this photodissociation reaction a photon of ultraviolet radiation is absorbed, never to be seen again. The reaction leaves behind a free oxygen atom, which will soon recombine with O2 in another three-way collision to create another ozone molecule, which will absorb another photon of ultraviolet radiation, and so on. The process will continue, with a photon of ultraviolet radiation being aborbed in each cycle od the raections (1) and (2) until some other chemical reaction intervenes to cause "odd oxygen" (i.e., an oxygen atom or an ozone molecule) to react with other chemicals in such a way as to form O2 as a by-product. The sequence of reactions in (1) and (2) os the mechanism by which the ozone layer shields the troposphere and life on the earth's surface from most of the harmful effects of ultraviolet radiation.
A small amount of potentially harmful radiation does get through at wavelengths right around 0.31 micron. It consists of photons that are not quite energetic enough to photodissociate ozone molecules as effectively, but still with enough energy to burn human skin and, with repeated exposure over an extended period of time, to substantially increase the risk of skin cancer, as evidenced by epidemiological statistics. rates of skin cancer are anomalously high in the tropics and subtropics, where the ozone layer is relatively thin and solar zenith angles are small so that sunlight passes through it directly, than at higher latitudes.
Ground based measurements of the total amount of ozone residing in a vertical column have been taken at a select group if stations since the 1920's. They are made by comparing the incoming radiation at two wavelengths near 0.31 microns: one within and the other just beyind the range in which effectively photodissociates ozone. In 1985, xxx published a paper reporting that total column ozone over an Antarctic station during springtime (late September, October, and early November) had been decreasing for nearly two decades and was down to about xx% of what it was during the 1950's. [LINK TO GRAPHIC] This finding was initially greeted with skepticism but was quickly verified by inspection of maps of total ozone based on satellite imagery, which were available since the 1970's. It's notable that this important scientific finding didn't result from the testing of a pre existing hypothesis: the discovery of the Antarctic 'ozone hole' was totally unexpected.
During the Antarctic springs since 1985 the ozone hole has continued to expand: total column ozone over Antarctica during those months has dropped from xxx Dobson Units (DU) in the 1950's to ~zzz DU in 1985 to ~xxx DU in the late 1990's. There have been incidences in which very low ozone amounts have been observed over southern Chle and Argentine during these months. [LINK TO GRAPHICS] Stratospheric ozone over higher latitudes (poleward of 45 N) of the Northern Hemisphere during wintertime is also beginning to decrease, but thus far the changes are far less dramatic and total column amounts remain in a range well above values typically observed in the tropics.
Three atmospheric chemists, Paul Crutzen, Sherry Rowland and xx Molina shared a Nobel prize in 199x for their detective work in linking the development of the ozone hole to the breakdown of industrially produced chlorofluorocarbon gases in the stratosphere. CFC's (commonly known as freons) are inert gases in which chlorine and fluorine (or bromine?) atoms are linked with carbon atoms to form inert compounds that serve a variety of purposes. Their most common use is in air conditioning, but for some years they were also widely used in aerosol spray cans and in styrofoam.
CFC's do not attack ozone directly. It is the free chlorine atoms that result from the breakdown of CFC's by ultraviolet radiation that do the damage through a pair of catalytic reactions
Cl + O3 -> ClO + O2
ClO + O -> Cl + O2
The net result of the two reactions is that an ozone molecule and an oxygen atom are converted into a pair of O2 molecules. The chlorine atom remains behind to do more damage: it has played the role of a catalyst.
The liberation of chlorine atoms through the breakdown
of CFC's requires multiple chemical reactions that can only occur
when the CFC's dissolve in microscopic cloud droplets droplets at temperatures
below -80 C. These
onditions are met only in the polar night
regions of the stratosphere. The other essential requirement is sunlight.
That's why the Antarctic ozone hole forms each year, not during winter,
but starting right around the equinox when the sun is just coming over
the horizon in the polar cap region.
Why is the loss of ozone so much more pronounced in the Antarctic than in the Arctic? The difference lies in the contrasting land-sea configurations in the Northern and Southern Hemispheres. The massive high latitude continents in the Northern Hemisphere induce strong planetary waves in the stratosphere which transport heat poleward, warming the polar cap region. As a result, temperatures drop below -80 C for only brief periods within patchy regions. In contrast, the Southern Hemisphere stratospheric winter wind patterns is much simpler and more consistent from day to day, with air circlulating around the pole in a strong westerly jet. The air within the polar night region tends to remain trapped there for weeks and sometimes even months, and hence has much more time to radiate away its heat and participate in the chemical reactions that set the stage for ozone destruction.
ozone layer and health:
http://www.gcrio.org/CONSEQUENCES/summer95/impacts.html
To address this question we need to consider a set of more specific questions:
1) How much are greenhouse gas concentrations likely to rise and how fast will it happen?
2) How much will the expected increases in greenhouse gas concentrations cause global-mean temperature to rise?
3) How will the warming of the planet impact local weather and climate?
4) How much will sea level rise?
5) How much will fossil fuel burning increase atmospheric concentrations of CO2?
6) What are the social, political and economic implications of all the above changes?
The is a wide range of uncertainty in the answers to all these questions, and the uncertainties become compounded as we move down the list. For example, how much sea-level will rise depends upon how much global-mean temperature rises, but it also depends upon how the continental ice sheets respond to that temperature rise.
These questions are being addressed using a number
of different kinds of numerical models together with information provided
by specialists in various fields. These questions are being addressed using
a number of different kinds ofnumerical models together with information
provided by specialists invarious fields. Here's a brief summary
of the kinds of models and types ofinformation that are being used to address
each of the questions:
1) geological surveys provide estimates of existing inventories of fossil fuels; models of the carbon cycle enable us to estimate how much of the fossil fuel that is burned will remain in the atmosphere and for how long; social scientists provide estimates of the rate of consumption of fossil fuels, making use of information provided by engineers and physicists concering alternative energy technologies,
2) simple 'back of the envelope' models provide crude estimates, but in order to represent the feedbacks as accurately as possible, complex global climate models of the atmosphere-ocean-land system are needed,
3) finer scale, atmospheric models with more detailed treatment of terrain and land sea geometry are 'nested' within the global models to provide more detailed local information in regions of mountainous terrain like most of the western U.S..
4) models of the thermohaline circulation tell us how deeply and how fast the warming will penetrate below the thermocline. For any predicted distribution of warming, the sea level rise it easy to estimate,
5) ecosystem models are used to predict how various species of plants and animals will respond to the changes in climate and to sea-level rises estimated in 3) and 4).
6) social and political implications are largely
speculative at this point. Economic models are used as a basis for quantifying
impacts of greengouse warming and comparing them with the costs of various
proposed mitigation strategies.
1) How much are greenhouse gas concentrations likely to rise and how fast will it happen?
We can place an upper limit upon atmospheric concentrations of carbon dioxide that could result from the burning of fossil fuels simply by envisioning a scenario (a hypothetical future) in which the entire global inventory of fossil fuels is consumed over a short period of time like a few hundred years.
The inventory of fossil fuels totals 5000 Gt of carbon: 500 in the form of oil, 500 in natural gas, and the remaining 4000 in coal. Oil shales are not included in the inventory under the assumption that mining them would be prohibitively expensive and environmentally destructive.
Models of the carbon cycle indicate that the oceans are capable of taking into solution a maximum of 1800 Gt of carbon, or about 1/3 of the fossil fuel inventory. The amount is set by self limiting chemical reactions: as additional CO2 dissolves, the oceans become more acidic, and as a result, they are less able to absorb additional CO2. The greening of the terrestrial biosphere could result in some additional sequestering of the carbon released into the atmosphere by the burning of fossil fuels, but the amount is modest. For the sake of simplicity, let's simply ignore it. In that case, if all the fossil fuels were burned instantaneously, 3200 Gt could be expected to remain in the atmosphere, boosting CO2 concentrations to 5-6 times their pre-industrial levels (580 Gt). Current projections of population growth and energy consumption indicate that present inventories of fossil fuels would be depleted in a few hundred years if they continue to be used as the primary energy source for human activity.
Carbonate formation by microscopic sea animals would gradually remove the excess CO2 from the oceans, allowing more atmospheric CO2 to dissolve, but this removal process takes place very slowly compared to amounts released into the atmosphere by the present and projected rates of consumption of fossil fuels. Model calculations indicate that it would take about 1000 year to remove 1/3 of the excess carbon from the atmosphere; another 1/3 years to remove 1/3 of the remainder, etc. Because of this ongoing removal of the excess carbon, slowing the rate of consumption of fossil fuels is bound to lower the ensuing peak in atmospheric concentrations. However, the removal rate is so gradual that slowing the rate of consumption, say by a factor of 2, would lower the peak atmospheric concentrations by only ~10%. The only way to significantly reduce peak concentrations is for humankind to stop burning fossil fuels well before the 5000 Gt carbon reservior is totally depleted.
Present atmospheric CO2 concentrations (750 Gt) are already 28% higher than their pre-industrial values. The typical 'scenarios' used in simulations of greenhouse warming in climate models, atmospheric CO2 concentrations double (relative to pre-industrial concentrations of 280 ppm) by the year 2100, and eventually triple quadruple, reaching levels ~1200 ppm a century ot two later. To hold peak concentrations below those levels, society would have to resort to energy sources other than fossil fuels within the next few generations.
For a more detailed discussion of energy economics and fossil fuel inventories, see Chapter 10 of the class notes from previous versions of this class.
For access to a wealth of information on energy consumption, see the web site for ENGR/Phys 341: http://swhite.me.washington.edu/~malte/engr341/ Accessible from that site is a lecture that Professor Philip Malte gave to the Fisheries 498 course on sustainable development, viewable with Adobe Acrobat. To get to it directly go to http://swhite.me.washington.edu/~malte/engr341/reserve/books.html and scroll to the bottom.
2) How much will the expected increases in greenhouse gas concentrations cause global-mean temperature to rise?
In support of the United Nations sponsored Intergovernmental Panel on Climate Change,(IPCC), a number of different climate models, developed by research groups in different countries have been run with CO2 concentrations increasing gradually through the 21st century to a level of twice the pre-industrial value (2 x 280 ppm = 560 ppm) in the year 2100. Included in the models are not only CO2, but also methane, nitrous oxide, CFC's and aerosols. The models attempt to simulate all known feedbacks (i.e., water vapor feedback, ice-albedo feedback, the various cloud-related feedbacks, and land vegetation feedback).
The estimated rise in global-mean temperature to the year 2100, as deduced from a large suite of experiments using models developed at many different research institutions, ranges from 1.0 C to 3.5 C (2-6 F) relative to pre-industrial values. But that's not the whole story. The models predict that even if atmospheric CO2 levels were held constant at 2 x pre-industrial levels from the year 2100 onward, global-mean temperature would continue to rise for another century or so until the temperatures of the deeper layers of the ocean had time to come into equilibrium with the warmer atmosphere. This vertical redistribution of heat within the oceans takes place by way of the thermohaline circulation. It takes several hundred years for parcels of water to make a complete circuit of the thermohaline 'conveyor belt'. Hence, if the doubled CO2 simulations are extended beyond the year 2100, global-mean temperature eventually rises to 1.5 to 6,0 C (3-10 F) from pre-industrial levels.
A doubling of atmospheric CO2 concentrations represents a rather optimistic scenario that presumes that the nations of the world will take major steps to reduce their dependence upon fossil fuels during the 21st century. Many of the climate modeling groups have also performed experiments for a less optimistic scenario in which CO2 concentrations would eventually quadruple. Based on these experiments it is estimated that quadrupling of atmospheric concentrations would raise global-mean temperature by approximately twice the amounts quoted in the previous paragraphs.
The large range of these numbers reflects the
large uncertainties in the feedbacks: hence, some of the models are more
sensitive to forcing by greenhouse gases than others. Given the crudeness
of the models, it's not inconceivable that the actual warming could even
fall outside this range of estimates.
3) How will the warming impact local weather and climate?
Temperature rises are expected to be
- larger and more rapid over land than over sea because of the lower heat capacity of the land (this is the bad news),
-larger in high latitudes than in low latitudes and larger in winter than during summer because of the ice-albedo feedback (this is the good news)
Growing seasons, as measured by the time interval between the last frost in spring and the first forst of autumn will trend to lengthen, especially at the higher latitudes.
The hydrological cycle should be more vigorous: on average, rainfall amounts are expected to increase, but this may not be true of all areas. In some of the models, warmer summer temperatures induced by greenhouse warming tend to dry out the soil in marginally arid regions such as the U.S. Great Plains, leading to local decreases in vegetation. Decreased vegetation tends to reduce the evaporation from the land surface in these regions, resulting in still higher afternoon temperatures, and lower relative humidities. These effects mimic what would happen if part of the land area in these regions were covered with pavement. As a result of the reduced evaporation, less moisture is available to feed summer rain events in these regions, and so rainfall is reduced. These positive 'land vegetation feedbacks' can make the local impacts of greenhouse much more serious in semi-arid regions than over the globe as a whole. This is why the IPCC Report mentioned the possibility of increased droughts as well as increased flooding in response to greenhouse warming.
Whether global warming would impact 'extreme weather events' such as hurricanes or tornadoes, or the occurrence of extreme heat or extreme cold is much more difficult to say. At this point, very few reputable scientists would make any claims (one way or the other) regarding changes in the frequency of severe storms. In terms of extreme temperatures, one should increase the incidence of temperatures above specified thresholds like 100 F and it should tend to decrease the incidence of temperatures below specified thresholds like 32 F. It hasn't been estabished that anything more dramatic than that would happen.
- in monsoon climates, the hottest months of the year tend to occur before the onset of the monsoon
- the tornado seaon in the central and southeastern U.S. is not in summer, but in spring
- the regions of the earth that are most vulnerable to hurricanes do not correpspond to the hottest parts of the tropics.
In terms of extreme temperatures, global warming should increase the incidence of temperatures above specified thresholds like 100 F and it should tend to decrease the incidence of temperatures below specified thresholds like 32 F, the limiting temperature for frost. There is, in fact, evidence of such changes during the 20th century. Shifts in the frequency of extreme temperatures can be made to sound quite dramatic. For example, lat us suppose that during the 20th century at some hypothetical place like Seattle, the the number of days with extremely hot temperatures was as follows
95-96 97-98
99-100 101-102 >102
30
15 5
1 0
Now let us suppose that temperatures during the 21st century were 2 F warmer than duting the 20th century, but that the climate was otherwise unchanged. In this case, all the temperatures the above table would be shifted toward the roght by one category:
97-98 99-100 101-102 >102
30 15
5 1
4) How much will sea level rise?
Based on the model estimates, sea level could rise by anywhere from 15 cm to a meter by the year 2100 if atmospheric CO2 concentrations double by that time. Assuming no further increase in CO2 concentrations, the sea level would rise by a nearly comparable amount in the 22nd century. This estimte To put these statistics into perspective, a 1 meter rise would be enough to inundate low lying coastal areas such as the Everglades, many of the barrier islands along the coast, most of the coral islands in the South Pacific and heavily populated coastal areas in Bangladesh. In Washington, Willapa Bay and other low lying coastal areas would be flooded, as well as areas in the south of Puget Sound including much of downtown Olympia.
The above estimates presume that there will not
be major changes in the continental ice sheets. Disaster scenarios
involving sudden sea level rises of many meters, flooding of coastal cities
are based on the premise that the ice sheets will begin to melt or that
segments of them will break loose and melt.
5) How will ecosystems, agriculture, fisheries and human health be impacted?
It's difficult to say in detail, since investigations of these effects have just recently begun. if global temperatures warm significantly, the range of plant and animal species should tend to migrate poleward with the temperatures, This migration will be much faster than the normal evolution of ecosystems, so the adjustement could cause problems such as incursions of 'exotic species' that have no natural predators. In mountainous terrain, species will tend to migrate toward higher altitude. Tropical species that are not tolerant of additional heat may not be able to survive the changes.
Agriculture in tropical climates will tend to suffer, and in high latitude climates such as those of Russia and Canada it should tend to prosper. In temperate climates such as the US, farmers should be able to adapt to the warming by planting more heat resistant crops and taking advantage of the longer growing season. Pests that are normally held in check by winter frostsmay be harder to control.
Tropical diseases such as malaria may become more
of a problem in middle latitudes. Populations vulnerable to extreme
heat and without access to air conditioning might find the temperature
increases unendurable.