| What is an APN? Acyl peroxy nitrates,
or APNs, are produced in the thermal equilibrium between organic peroxy
radicals and nitrogen dioxide:
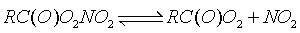 Organic peroxy radicals are formed by the gas-phase oxidation of a variety of volatile organic compounds (VOCs); as such, the specific structure of the R group can be indicitive of the nature and origin of the VOC precursor. For example, peroxypropionyl nitrate (PPN) is primarily anthropogenic in origin, while peroxymethacryloyl nitrate (MPAN) is thought to be largely a biproduct of the oxidation of unsaturated biogenic VOCs. Peroxyacetyl nitrate (PAN), the most abundant APN, is derived from both anthropogenic and biogenic VOCs.  Consider the
following:
- NOx
(= NO + NO2),
which is emitted in areas of biomass burning and fossil fuel
combustion, is a key participant in the catalytic production of ozone
- There is a growing concern that NOx emissions in a rapidly industrializing Asia are enhancing ozone production in remote regions of the Pacific Northwest - The lifetime of NOx in the boundary layer is on the order of a day, which is generally shorter than the timescale for venting to the free troposphere
Thus
the formation of stable, insoluble NOx
reservoirs determines its long range transport
 Some NOx
is converted into the acyl peroxy nitrate reservoir, the lifetime of which
increases dramatically at low temperatures. APNs, along with
other long-lived pollutants like CO, can then be transported far from
source regions. Upon subsidence, these airmasses
warm and the APN equilibrium shifts back to
the right, resulting in a release of NOx that feeds
catalytic ozone production. In short, APNs act as the "transport
vehicle" by
which NOx travels to remote areas.
How do we measure APNs? Our laboratory
employs chemical
ionization mass spectrometry (CIMS) to measure a suite of APNs present
in the atmosphere. The detection scheme, based on work by Villalta and Howard (J.
Phys. Chem. 1996, 100) and Slusher, et al. (J. Geophys. Res. 2004, D19315)
involves three steps:
1)Thermal dissociation of APNs into NO2 and RC(O)O2 radicals; 2)Reaction of the peroxy radicals with iodide anions to generate RC(O)O-; 3)Detection of these ions by a customized quadrupole mass spectrometer system. A standard field setup is shown below. Currently, the CIMS achieves a detection limit of ~5 pptv for a one-second measurement of PAN (S/N = 2). 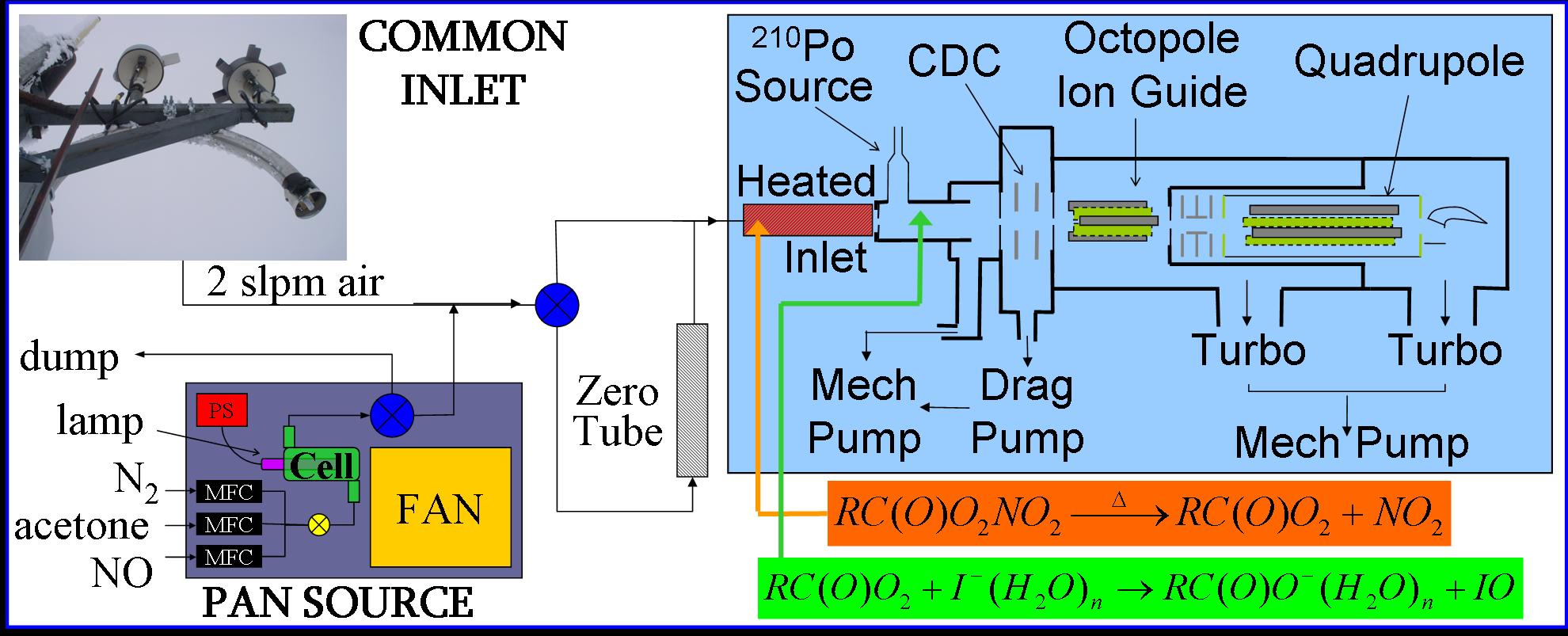 Since we retain the "R" group in this scheme, the CIMS technique provides the advantage of making speciated measurements rather than just having a "sum of APNs." Such measurements can be useful for determining pollution sources, estimating the age of a pollution plume and identifying previously undetected APNs in both urban and forested areas. |